About Biopol®
BIOPOL® Polymers are water soluble crosslinked Polyacrylic Acid. It is offered as fluffy dry
white powder.
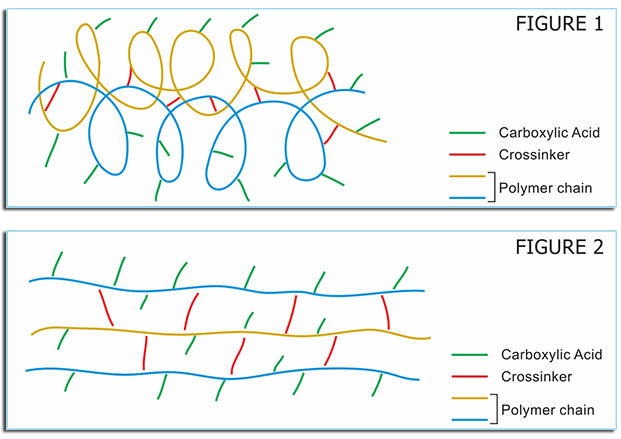
BIOPOL® polymers have been used for over 50 years for various applications with the highest quality carbomers on the market. Their application in products as rheology modifiers, tablet binders, suspension stabilizers, thickeners, extended release polymers, mucoadhesive aids, bioavailability enhancers have increased their demand as they are versatile and their ease of use has created its demand in Pharmaceutical, Nutraceutical, Personal Care & Allied Industrial applications. Grades within the BIOPOL® family are chemically similar in terms of the fact that they are all high molecular weight, crosslinked polyacrylic acid polymers. However, these gel forming polymers differ by their chemical crosslinking and can be grouped
into 3 categories.